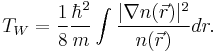Thomas–Fermi model
The Thomas–Fermi (TF) model,[1][2] named after Llewellyn Thomas and Enrico Fermi, is a quantum mechanical theory for the electronic structure of many-body systems developed semiclassically shortly after the introduction of the Schrödinger equation.[3] It stands separate from wave function theory as being formulated in terms of the electronic density alone and as such is viewed as a precursor to modern density functional theory. The TF model is correct only in the limit of an infinite nuclear charge. Using the approximation for realistic systems yields poor quantitative predictions, even failing to reproduce some general features of the density such as shell structure in atoms and Friedel oscillations in solids. It has, however, found modern applications in many fields through the ability to extract qualitative trends analytically and with the ease at which the model can be solved. The kinetic energy expression of Thomas-Fermi theory is also used as a component in more sophisticated density approximation to the kinetic energy within modern orbital-free density functional theory.
Working independently, Thomas and Fermi used this statistical model in 1927 to approximate the distribution of electrons in an atom. Although electrons are distributed nonuniformly in an atom, an approximation was made that the electrons are distributed uniformly in each small volume element ΔV (i.e. locally) but the electron density 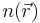 can still vary from one small volume element to the next.
can still vary from one small volume element to the next.
Contents |
Kinetic energy
For a small volume element ΔV, and for the atom in its ground state, we can fill out a spherical momentum space volume Vf up to the Fermi momentum pf , and thus,[4]
where 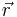 is a point in ΔV.
is a point in ΔV.
The corresponding phase space volume is
The electrons in ΔVph are distributed uniformly with two electrons per h3 of this phase space volume, where h is Planck's constant.[5] Then the number of electrons in ΔVph is
The number of electrons in ΔV is
where 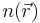 is the electron density.
is the electron density.
Equating the number of electrons in ΔV to that in ΔVph gives,
The fraction of electrons at 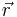 that have momentum between p and p+dp is,
that have momentum between p and p+dp is,
Using the classical expression for the kinetic energy of an electron with mass me, the kinetic energy per unit volume at 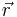 for the electrons of the atom is,
for the electrons of the atom is,
where a previous expression relating 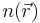 to
to 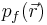 has been used and,
has been used and,
Integrating the kinetic energy per unit volume 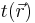 over all space, results in the total kinetic energy of the electrons,[6]
over all space, results in the total kinetic energy of the electrons,[6]
This result shows that the total kinetic energy of the electrons can be expressed in terms of only the spatially-varying electron density 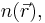 according to the Thomas–Fermi model. As such, they were able to calculate the energy of an atom using this expression for the kinetic energy combined with the classical expressions for the nuclear-electron and electron-electron interactions (which can both also be represented in terms of the electron density).
according to the Thomas–Fermi model. As such, they were able to calculate the energy of an atom using this expression for the kinetic energy combined with the classical expressions for the nuclear-electron and electron-electron interactions (which can both also be represented in terms of the electron density).
Potential energies
The potential energy of an atom's electrons, due to the electric attraction of the positively charged nucleus is,
where 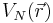 is the potential energy of an electron at
is the potential energy of an electron at 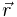 that is due to the electric field of the nucleus. For the case of a nucleus centered at
that is due to the electric field of the nucleus. For the case of a nucleus centered at 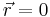 with charge Ze, where Z is a positive integer and e is the elementary charge,
with charge Ze, where Z is a positive integer and e is the elementary charge,
The potential energy of the electrons due to their mutual electric repulsion is,
Total energy
The total energy of the electrons is the sum of their kinetic and potential energies,[7]
Inaccuracies and improvements
Although this was an important first step, the Thomas-Fermi equation's accuracy is limited because the resulting expression for the kinetic energy is only approximate, and because the method does not attempt to represent the exchange energy of an atom as a conclusion of the Pauli principle. A term for the exchange energy was added by Dirac in 1928.
However, the Thomas-Fermi-Dirac theory remained rather inaccurate for most applications. The largest source of error was in the representation of the kinetic energy, followed by the errors in the exchange energy, and due to the complete neglect of electron correlation.
In 1962, Edward Teller showed that Thomas-Fermi theory cannot describe molecular bonding – the energy of any molecule calculated with TF theory is higher than the sum of the energies of the constituent atoms. More generally, the total energy of a molecule decreases when the bond lengths are uniformly increased.[8][9][10][11] This can be overcome by improving the expression for the kinetic energy.[12]
The Thomas-Fermi kinetic energy can be improved by adding to it the Weizsäcker (1935) correction:[13]
See also
References
- R. G. Parr and W. Yang (1989). Density-Functional Theory of Atoms and Molecules. New York: Oxford University Press. ISBN 978-0-19-509276-9.
- N. H. March (1992). Electron Density Theory of Atoms and Molecules. Academic Press. ISBN 978-0-12-470525-8.
- N. H. March (1983). "1. Origins – The Thomas–Fermi Theory". In S. Lundqvist and N. H. March. Theory of The Inhomogeneous Electron Gas. Plenum Press. ISBN 978-0306412073.
Footnotes
- ^ Thomas, L. H. (1927). "The calculation of atomic fields". Proc. Cambridge Phil. Soc. 23 (5): 542–548. Bibcode 1927PCPS...23..542T. doi:10.1017/S0305004100011683.
- ^ Fermi, Enrico (1927). "Un Metodo Statistico per la Determinazione di alcune Prioprietà dell'Atomo". Rend. Accad. Naz. Lincei 6: 602–607. http://babel.hathitrust.org/cgi/pt?seq=339&view=image&size=100&id=mdp.39015001321200&u=1&num=278.
- ^ Schrödinger, Erwin (December 1926). "An Undulatory Theory of the Mechanics of Atoms and Molecules" (PDF). Phys. Rev. 28 (6): 1049–1070. Bibcode 1926PhRv...28.1049S. doi:10.1103/PhysRev.28.1049. http://home.tiscali.nl/physis/HistoricPaper/Schroedinger/Schroedinger1926c.pdf.
- ^ March 1992, p.24
- ^ Parr and Yang 1989, p.47
- ^ March 1983, p. 5, Eq. 11
- ^ March 1983, p. 6, Eq. 15
- ^ Teller, E. (1962). "On the Stability of molecules in the Thomas-Fermi theory". Rev. Mod. Phys. 34 (4): 627–631. Bibcode 1962RvMP...34..627T. doi:10.1103/RevModPhys.34.627.
- ^ Balàzs, N. (1967). "Formation of stable molecules within the statistical theory of atoms". Phys. Rev. 156 (1): 42–47. Bibcode 1967PhRv..156...42B. doi:10.1103/PhysRev.156.42.
- ^ Lieb, Elliott H.; Simon, Barry (1977). "The Thomas-Fermi theory of atoms, molecules and solids". Adv. In Math. 23 (1): 22–116. doi:10.1016/0001-8708(77)90108-6.
- ^ Parr and Yang 1989, pp.114–115
- ^ Parr and Yang 1989, p.127
- ^ Weizsäcker, C. F. v. (1935). "Zur Theorie der Kernmassen". Zeitschrift für Physik 96 (7-8): 431–458. Bibcode 1935ZPhy...96..431W. doi:10.1007/BF01337700.
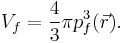
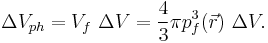
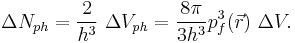
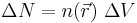
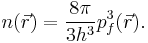
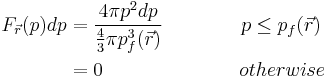
![\begin{align}
t(\vec{r}) & = \int \frac{p^2}{2m_e} \ n(\vec{r}) \ F_\vec{r} (p) \ dp \\
& = n(\vec{r}) \int_{0}^{p_f(\vec{r})} \frac{p^2}{2m_e} \ \ \frac{4 \pi p^2 } {\frac{4}{3} \pi p_f^3(\vec{r})} \ dp \\
& = C_F \ [n(\vec{r})]^{5/3}
\end{align}](/2012-wikipedia_en_all_nopic_01_2012/I/2bdc32c0de99e7d5b4f9d3c903dd42cd.png)
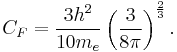
![T=C_F\int [n(\vec{r})]^{5/3}\ d^3r \ .](/2012-wikipedia_en_all_nopic_01_2012/I/5b882b0c0b42617723a0308fafa09fe1.png)
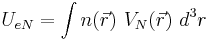
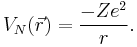
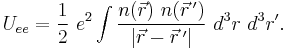
![\begin{align}
E & = T \ %2B \ U_{eN} \ %2B \ U_{ee} \\
& = C_F\int [n(\vec{r})]^{5/3}\ d^3r \ %2B \int n(\vec{r}) \ V_N(\vec{r}) \ d^3r \ %2B \ \frac{1}{2} \ e^2 \int \frac{n(\vec{r}) \ n(\vec{r} \, ')} {\left\vert \vec{r} - \vec{r} \, ' \right\vert } \ d^3r \ d^3r' \\
\end{align}](/2012-wikipedia_en_all_nopic_01_2012/I/42213f8ab453bb30a9aff5411d8c8d8f.png)